Pogil electron configuration and orbitals, a fundamental concept in chemistry, provide a comprehensive understanding of the arrangement and behavior of electrons within atoms. This article delves into the intricacies of electron configuration, the shapes and properties of atomic orbitals, and their profound influence on the chemical properties and bonding behavior of elements.
Electron configuration, a roadmap to the distribution of electrons in an atom’s energy levels, governs the chemical reactivity and bonding tendencies of elements. Orbitals, the three-dimensional regions where electrons reside, dictate the geometry and properties of molecules. Together, electron configuration and orbitals form the cornerstone of our understanding of chemical bonding and the periodic table.
Electron Configuration

Electron configuration refers to the arrangement of electrons within an atom. It describes the distribution of electrons among the various energy levels and orbitals surrounding the atomic nucleus.
The electron configuration of an element can be determined by its atomic number, which represents the number of protons in its nucleus. The number of electrons in an atom is typically equal to the atomic number, making the atom electrically neutral.
Periodic Trends in Electron Configuration
Electron configuration exhibits periodic trends across the periodic table. Elements in the same group (vertical column) have similar electron configurations, particularly in their outermost energy level. This similarity in electron configuration gives rise to similar chemical properties within each group.
Moving from left to right across a period (horizontal row), the electron configuration changes gradually as electrons are added to the outermost energy level. This results in a gradual change in chemical properties, with elements becoming more metallic towards the left and more non-metallic towards the right.
Orbitals

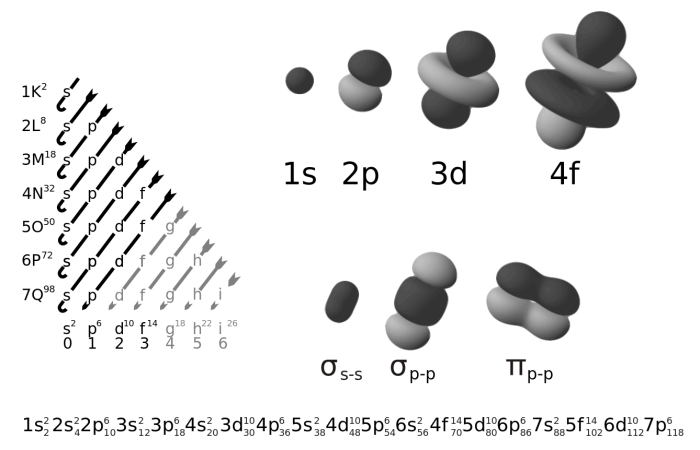
Atomic orbitals are mathematical functions that describe the wave-like behavior of electrons within an atom. Each orbital represents a region of space around the nucleus where an electron is likely to be found.
There are different types of orbitals, designated by the letters s, p, d, and f. These orbitals have distinct shapes and properties:
- s orbitals: Spherical in shape, with the highest electron density near the nucleus.
- p orbitals: Dumbbell-shaped, with two lobes oriented along the x, y, or z axis.
- d orbitals: More complex shapes, with five different orientations.
- f orbitals: Even more complex shapes, with seven different orientations.
Relationship between Electron Configuration and Orbital Occupancy, Pogil electron configuration and orbitals
The electron configuration of an atom determines the occupancy of its orbitals. Electrons occupy orbitals in a specific order based on their energy levels and the Pauli exclusion principle, which states that no two electrons can occupy the same quantum state.
The lowest energy orbitals are filled first, followed by higher energy orbitals. Within each energy level, the orbitals are filled in order of increasing angular momentum (l). For example, the 2p orbitals are filled before the 3s orbitals.
Periodic Table: Pogil Electron Configuration And Orbitals
The periodic table is organized based on the electron configurations of the elements. Elements in the same group have similar electron configurations, resulting in similar chemical properties.
The periodic table can be divided into four blocks based on the outermost electron configuration:
- s-block: Elements with electrons in the outermost s orbital (Groups 1 and 2).
- p-block: Elements with electrons in the outermost p orbital (Groups 13-18).
- d-block: Elements with electrons in the outermost d orbital (Groups 3-12).
- f-block: Elements with electrons in the outermost f orbital (Lanthanides and Actinides).
Relationship between Electron Configuration and Chemical Properties
The electron configuration of an element can be used to predict its chemical properties. For example, elements in the same group tend to have similar chemical properties due to their similar electron configurations.
The outermost electrons, known as valence electrons, are primarily responsible for chemical bonding. The number and arrangement of valence electrons determine the element’s reactivity and the types of bonds it can form.
Chemical Bonding
Electron configuration plays a crucial role in chemical bonding. The valence electrons of atoms interact with each other to form chemical bonds, which hold atoms together to create molecules and compounds.
There are three main types of chemical bonds:
- Ionic bonds: Formed between atoms that have significantly different electronegativities, resulting in the transfer of electrons from one atom to another.
- Covalent bonds: Formed between atoms that share electrons, creating a strong bond between the atoms.
- Metallic bonds: Formed between metal atoms, where valence electrons are delocalized and can move freely throughout the metal.
Electron Configuration and Bond Type
The electron configuration of an atom can be used to predict the type of bond it will form. For example, elements with low ionization energies and high electron affinities tend to form ionic bonds, while elements with similar electronegativities tend to form covalent bonds.
Applications
Understanding electron configuration and orbitals has numerous practical applications in various fields:
- Chemistry: Predicting chemical properties, designing new materials, and understanding chemical reactions.
- Materials science: Developing new materials with specific properties, such as semiconductors and superconductors.
- Nanotechnology: Designing and manipulating materials at the nanoscale, where electron configuration plays a crucial role in determining the properties of nanostructures.
Electron Configuration and Advancements
Understanding electron configuration has led to significant advancements in these fields. For example, the development of transistors, the foundation of modern electronics, relies heavily on the manipulation of electron configurations in semiconductors.
In nanotechnology, the ability to control electron configurations at the nanoscale has enabled the development of novel materials with unique properties, such as carbon nanotubes and graphene.
Common Queries
What is electron configuration?
Electron configuration describes the arrangement of electrons in the energy levels and orbitals of an atom.
How do orbitals influence chemical bonding?
Orbitals determine the geometry and strength of chemical bonds by dictating the overlap and interaction of electron clouds.